Abstract
The enantioselective construction of C–CF2R (R: alkyl or fluoroalkyl) bonds has attracted the attention of synthetic chemists because of the importance of chiral fluorinated compounds in life and materials sciences. Catalytic asymmetric fluoroalkylation has mainly been realized under organocatalysis and Lewis acid catalysis, with substrates limited to carbonyl compounds. Few examples using transition-metal catalysis exist, owing to side reactions including decomposition and isomerization of fluoroalkylating reagents. Herein we report umpolung asymmetric difluoroallylation of hydrazones with 3-bromo-3,3-difluoropropene (BDFP) under palladium catalysis. Difluoroallylation products having quaternary chiral carbon centers are afforded in good yields with high α/γ- and enantioselectivities. The usefulness of the reaction products is demonstrated and an inner-sphere mechanism of the reaction is proposed. The use of chiral N-heterocyclic carbene as ligand is the key for the selectivities as well as the productivity of the reaction.
Similar content being viewed by others
Introduction
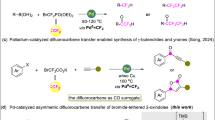
The site-selective introduction of fluorinated groups into organic molecules has received extensive attention due to the important applications of fluorinated compounds in pharmaceuticals, agrochemicals, and materials sciences (Fig. 1)1,2. Over the past decades, tremendous efforts have been made in synthetic chemistry of fluorine-containing compounds, most of which focus on the direct fluoroalkylation of aromatic frameworks3,4,5, while less attention has been paid to the fluoroalkylation of aliphatic substrates6. Asymmetric fluoroalkylation has mainly been developed under organocatalysis7,8,9,10,11,12,13,14,15,16,17 and Lewis acid catalysis18,19,20, the substrates being limited to carbonyl compounds (Fig. 2a). Although transition-metal catalysis has been a powerful tool in organic synthesis, few successful examples in synthetic fluorine chemistry have appeared by using this strategy (Fig. 2b)21,22,23,24,25 because it is prone to generate side reactions, such as decomposition or isomerization of fluoroalkylating reagents. How to construct the C–CF2R bonds at the stereogenic center enantioselectively and efficiently under transition-metal catalysis to meet the increased demanding of life and materials sciences should be a crucial issue to be addressed.
a Asymmetric fluoroalkylation under organocatalysis and Lewis acid catalysis. b Asymmetric fluoroalkylation under transition-metal catalysis. c Regioselectivity in the reaction of nucleophiles with BDFP. d Palladium-catalyzed umpolung regio- and enantioselective allylation of hydrazones with BDFP. The circles with different color = different substituents.
We are interested in the introduction of gem-difluoroallyl group into organic molecules in a catalytic asymmetric manner, because the transformations of its carbon-carbon double bond can lead to diversified chiral difluoroalkylated compounds, which have important applications in pharmaceuticals (Fig. 1). We have developed a palladium-catalyzed gem-difluoroallylation of arylborons with BDFP26 and some methodologies in palladium-catalyzed asymmetric allylic alkylation27,28,29,30. We questioned whether BDFP31,32 could be used in palladium-catalyzed asymmetric gem-difluoroallylation. One of the crucial issues in this process is the regioselectivity (α/γ-selectivity), in addition to the challenge of constructing C-CF2R bond enantioselectively, because previous reports demonstrated that γ-substitution of BDFP is a favorable pathway for the reaction with carbon or nitrogen nucleophile33 (Fig. 2c).
Herein, we disclose our preliminary results of our investigations on a palladium-catalyzed asymmetric umpolung gem-difluoroallylation of hydrazones using a newly synthesized chiral NHC as the ligand, affording gem-difluoroallylated products bearing a quaternary chiral carbon center with high α- and enantioselectivities (Fig. 2d)34,35,36,37,38. A Pd-mediated [3,3]-reductive elimination process (inner-sphere mechanism) is proposed for the high selecitivities37,38,39,40,41,42,43,44,45,46. The resulting products are easily converted into some other chiral fluoro-containing products, including chiral amines, which play an important role in medicinal chemistry47,48,49.
Results
Influence of the reaction parameters on the reaction
At the beginning of our investigation, we carried out the reaction of 2-methyl-1-phenylpropan-1-one with BDFP (2a) by using Pd/PPh3 as the catalyst. However, only γ-substituted product was produced (Fig. 3a). From the literatures and our own experiences, the regiochemistry in Pd-catalyzed allylic alkylation reaction could be different when the reaction proceeds via inner- or outer-sphere mechanism40,41,42,43,44,45,46. Thus, 1,3-bis(2,6-di-i-propylphenyl)imidazolidine-2-ylidene (SIPr), a NHC ligand previously used by us in Pd-catalyzed allylic alkylation of ketones via inner-sphere mechanism successfully, was tested as the ligand45,46. However, the reaction failed to proceed (Fig. 3b). Considering that enolate and aza-allyl anion are formed during the reaction of ketones and imines as nucleophile in Pd-catalyzed allylic alkylation44,45,46, and hydrazone has similar structure, we envisioned that hydrazone may be a suitable nucleophile for this allylation reaction. Pleasantly, allylation products were obtained in 86% yield with a ratio of 3a/4a/5a = 81/0/19 by the reaction of hydrazone 1a with BDFP (2a) using [Pd(C3H5)Cl]2 and SIPr as the catalyst (Table 1, entry 1). Obviously, the N-allylation product 5a was obtained via an SN2′ reaction with nitrogen as the nucleophile because 5a could be obtained without L and Pd (Table 1, entry 2), while α- and γ-substituted products 3a and 4a are umpolung allylic alkylation products. However, the chemoselectivity (C-/N-allylation) and the reaction efficiency were lower. To improve the chemoselectivity and to synthesize 3a enantioselectively, a series of ligands were tested (Table 1, Fig. 4). Phosphine ligands led to γ-substituted 4a as major product or sole formation of 5a (Table 1, entries 3 and 4). However, chiral NHC ligand L1 could provide allylation products in 48% yield with 50/0/50 ratio of 3a/4a/5a and 56:44 er for 3a (Table 1, entry 5). The yield increased to 93% with 57/7/36 ratio of 3a/4a/5a and 68.5:31.5 er for 3a by using a bulkier L2 as the ligand (Table 1, entry 6). The er value of 3a could increase further to 90.5:9.5 when L3 possessing an adamantyl (Ad) as one of the substituents on nitrogen was used (Table 1, entry 7). No γ-substitution product 4 was produced under these reaction conditions, but chemoselectivity was poor. No further improvement in chemo- and enantioselectivities was observed when NHC ligands L4–L7 with different substituents on nitrogen were used (Table 1, entries 8–11). Based on the structures of L3 and L7, we also designed and synthesized new NHC ligand L8. When it was used, the er increased a little, but the chemoselectivity was still low (Table 1, entry 12). To improve the efficiency and the selectivities of the reaction, the impact of other parameters on the reaction were investigated by using L8 as the ligand (Table 1). The results showed that the solvents we screened slightly affected the efficiency and the selectivities of the reaction (Table 1, entries 13–15), while the base has great influences on them. The reaction delivered the products in 86% yield with 57/0/43 ratio of 3a/4a/5a and 93.5:6.5 er for 3a when LiHMDS [HMDS: bis(trimethylsilyl)amide] was used as the base (Table 1, entry 12). Worse results were obtained if NaHMDS and KHMDS were the base (Table 1, entries 16 and 17). The efficiency and the selectivities were greatly improved, however, yield being 96%, the ratio of 3a/4a/5a being >99:0:1 with 95:5 er for 3a, if lithium diisopropylamide (LDA) was the base (Table 1, entry 18). Similar results were obtained by using n-BuLi as the base (Table 1, entry 19). If the reaction ran at lower temperature, similar enantioselectivity was observed, but the yield and the chemoselectivity were poorer (Table 1, entry 20). As comparison, the reaction with allyl bromide was also tested under the reaction condition of entry 18, Table 1. Only 24% yields of umpolung allylation and N-allylation products were obtained in a ratio of 27:73 with 86.5:13.5 er for umpolung product (not showed in Table 1). These results might reflect the importance of the difluoromethylene moiety of 2a regarding the efficiency and the selectivities of the reaction.
Structures of NHC ligands L1–L8 tested in Table 1.
Substrate scope
With the viable reaction conditions in hand, the substrate scope of the reaction was investigated (Fig. 5). It can be seen that a wide range of α-substituted products 3 with R1 as phenyl bearing electron-withdrawing groups (3b, 3c, 3 f, 3 g) or electron-donating groups (3d, 3e, 3h, 3i, 3k, 3m) at m- or p-position were afforded in 67–96% yields with 86:14-96:4 er. The substituent R1 of compounds 3 can also be naphthyl and 2-indolyl (3l and 3n), the enantioselectivities being 94:6 and 95:5 er, respectively, but the yield for 3n was a little bit lower. However, the reaction was sensitive to the steric effect, substrate with R1 as o-substituted phenyl failed to deliver the product (3j). Hydrazones derived from tetralone and its derivatives as well as from α, β-unsaturated ketones were also suitable substrates, delivering 3p–s and 3t–v as the products in high yields and high enantioselectivities. The hydrazone derived from propiophenone also underwent the current palladium-catalyzed process smoothly to provide product 3o in 90% with 93:7 er. Noteworthy is that the aliphatic hydrazones were also suitable substrate, affording α-substituted products 3x and 3y with Me, i-Pr and Me, Et as the substituents in 50% and 74% yields with 96:4 and 88:12 er, respectively. It was found that the presence of phenyl group on nitrogen should be important, the reaction failed to afford allylation product if N-tert-Bu hydrazone 1aa was the substrate (Fig. 6a). Only trace products were afforded when hydrazone 1z derived from cyclopropanecarbaldehyde was the substrate (Fig. 6b) although α-substituted product in 75% yield was obtained if SIPr·HCl was used as the ligand (not showed in Fig. 6). The reactions of hydrazone 1a with substituted BDFP, (E)-1-bromo-1,1-difluorooct-2-ene (2b) and (3-(bromodifluoromethyl)but-3-en-1-yl)benzene (2c), were also unsuccessful, only N-allylation with γ-selectivity and trace α-substituted products were observed respectively (Fig. 6c and d).
a Palladium-c-atalyzed reaction of N-tert-Bu hydrazone 1aa with BDFP. b Palladium-catalyzed reaction of hydrazone 1z with BDFP using L8 as the ligand. c Palladium-catalyzed reaction of hydrazone 1a with (E)-1-bromo-1,1-difluorooct-2-ene (2b). d Palladium-catalyzed reaction of hydrazone 1a with (3-(bromodifluoromethyl)but-3-en-1-yl)benzene (2c).
Synthetic applications
To demonstrate the utility of the methodology, various conversions of the reaction products were carried out (Fig. 7a). The N=N and C=C bonds of difluoroallylation product 3a were transformed into NH2 and C–C–OH groups, respectively, keeping er ratio of product 7 unchanged. The Heck reaction of 3a produced the desired product 9 in 65% yield with >20:1 E/Z ratio and 95:5 er. Treatment of 3a with B2pin2 under copper catalysis followed by oxidation provided mono-fluoro allylalcohol 8 in 80% yield with 96:4 er (Fig. 7a). Since these products such as fluorinated amino alcohols have important applications in medicinal chemistry47,48,49, this protocol provides an efficient route for applications in drug discovery and development. One-mmol scale reaction also proceeded to deliver similar results (Fig. 7b). The absolute configuration of product 7 was determined as (S) by X-ray diffraction analysis of its single crystal (Fig. 7).
Mechanistic study
It should be possible that the reaction of BDFP under transition-metal catalysis proceeds via radical mechanism50. Thus, control experiments by using radical clock probe 10 and TEMPO 11 were carried out (Fig. 8), from which the radical mechanism could be ruled out. In Pd-catalyzed allylic alkylation, there are two ways, inner- or outer-sphere processes, to form allylation products. In our previous works, the reactions using PPh3 as the ligand proceeded via outer-sphere mechanism to afford linear products predominantly, while that using NHC as the ligand underwent inner-sphere process to provide branched products45,46. Based upon these results and the literature reports37,38,39,43,44,45 as well as the present observations, the inner-sphere mechanism could be proposed for our present study, that is, the reaction proceeded through the attack of the nitrogen anion of hydrazone on the Pd of Pd-π difluoroallyl complex, followed by Pd-mediated [3,3]-reductive elimination (Fig. 9a). With this proposal and the results from the reaction using N-tert Bu hydrazone 1aa as the reagent (Fig. 6a), the possible transition states of the reaction could be proposed, in which π-π stacking between ligand and phenyl group on nitrogen of hydrazone should play the role, and products in (S)-configuration would be obtained via favored transition state TS2 (Fig. 9b). It should be noted that the detailed reaction mechanism should be studied further.
In conclusion, a regio- and enantioselective gem-difluoroallylation of hydrazones was realized under Pd/NHC catalysis in an umpolung manner. The usefulness of the protocol was demonstrated. A rational inner-sphere mechanism was proposed. The use of bulky chiral NHC ligand overcomes the challenges in chemo-, regio-, and enantioselective gem-difluoroallylation of hydrazones, paving a way for the catalytic asymmetric synthesis of fluorinated compounds. Detailed investigation of the reaction mechanism as well as the exploration of further applications of BDFP in asymmetric fluoroalkylations are underway.
Methods
General procedure for gem-difluoroallylation of hydrazones
A dry Schlenk tube was flame dried and flushed with Argon. Hydrazone 1 (0.4 mmol) and toluene (2.0 mL) were added into the dry Schlenk tube. LDA (1.0 M in THF, 0.4 mL, 0.4 mmol) was added at 0 °C and stirred at room temperature for 30 min. In a separated flask, [Pd(C3H5)Cl]2 (1.83 mg, 0.005 mmol), L8 (7.1 mg, 0.01 mmol) and toluene (1.0 mL) were mixed, followed by addition of t-BuOK (1.0 M in THF, 25 μL, 0.025 mmol) at rt. The resulting mixture was stirred at room temperature for 30 min, then added to the hydrazone solution. The BDFP 2 (31.5 mg, 0.2 mmol) and toluene (1.0 mL) was then added and the mixture was stirred at room temperature. After the reaction was completed, the reaction mixture was quenched by H2O (0.3 mL). The regio- and diastereoselectivities were then determined by 19F NMR spectroscopy by using trifluoromethylbenzene (24 μL) as an internal standard. After this analysis, the crude reaction mixture was dried (anhydrous Na2SO4) and then filtered through a 0.5 inch plug of silica gel (eluting with AcOEt) to remove the solid. The crude reaction mixture was concentrated under reduced pressure and then purified by preparative TLC (petroleum ether/ethyl acetate = 50/1) to afford products.
Data availability
Detailed experimental procedures and characterization of compounds as well as NMR and HPLC spectra can be found in the Supplementary Information. The X-ray crystallographic coordinates for structure reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number CCDC 2032248. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Wang, J. et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade. Chem. Rev. 114, 2432–2506 (2016).
Meanwell, N. A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 61, 5822–5880 (2018).
Furuya, T., Kamlet, A. S. & Ritter, T. Catalysis for fluorination and trifluoromethylation. Nature 473, 470–477 (2011).
Tomashenko, O. A. & Grushin, V. V. Aromatic trifluoromethylation with metal complexes. Chem. Rev. 111, 4475–4521 (2011).
Feng, Z., Xiao, Y.-L. & Zhang, X. Transition-metal (Cu, Pd, Ni)-catalyzed difluoroalkylation via cross-coupling with difluoroalkyl halides. Acc. Chem. Res. 51, 2264–2278 (2018).
Ma, J.-A. & Cahard, D. Update 1 of: asymmetric fluorination, trifluoromethylation, and perfluoroalkylation reactions. Chem. Rev. 108, PR1–PR43 (2008).
Shibata, N., Mizuta, S. & Kawai, H. Recent advances in enantioselective trifluoromethylation reactions. Tetrahedron Asymmetry 19, 2633–2644 (2008).
Yang, X., Wu, T., Phipps, R. J. & Toste, F. D. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 115, 826–870 (2015).
Iseki, K., Nagai, T. & Kobayashi, Y. Asymmetric trifluoromethylation of aldehydes and ketones with trifluoromethyltrimethylsilane catalyzed by chiral quaternary ammonium fluorides. Tetrahedron Lett. 35, 3137–3138 (1994).
Iseki, K. et al. Asymmetric aldol addition of aldehydes to a difluoroketene silyl acetal catalyzed by chiral Lewis acids. Tetrahedron 53, 10271–10280 (1997).
Kawai, H., Kusuda, A., Nakamura, S., Shiro, M. & Shibata, N. Catalytic enantioselective trifluoromethylation of azomethine imines with trimethyl(trifluoromethyl)silane. Angew. Chem. Int. Ed. 48, 6324–6327 (2011).
Kashikura, W., Mori, K. & Akiyama, T. Chiral phosphoric acid catalyzed enantioselective synthesis of β-Amino-α, α-difluoro carbonyl compounds. Org. Lett. 13, 1860–1863 (2011).
Liu, Y.-L., Yu, J.-S. & Zhou, J. Catalytic asymmetric construction of stereogenic carbon centers that feature a gem-difluoroalkyl group. Asian J. Org. Chem. 2, 194–206 (2013).
Zhang, P. & Wolf, C. Catalytic enantioselective difluoroalkylation of aldehydes. Angew. Chem. Int. Ed. 52, 7869–7873 (2013).
Nagib, D. A., Scott, M. E. & MacMillan, D. W. C. Enantioselective α-trifluoromethylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 131, 10875–10877 (2009).
Furukawa, T. et al. Organocatalyzed regio- and enantioselective allylic trifluoromethylation of Morita−Baylis−Hillman adducts using Ruppert−Prakash reagent. Org. Lett. 13, 3972–3975 (2011).
Li, Y. et al. Room temperature asymmetric allylic trifluoromethylation of Morita−Baylis−Hillman carbonates. Org. Lett. 13, 6082–6085 (2011).
Allen, A. E. & MacMillan, D. W. C. The productive merger of iodonium salts and organocatalysis: a non-photolytic approach to the enantioselective α-trifluoromethylation of aldehydes. J. Am. Chem. Soc. 132, 4986–4987 (2010).
Deng, Q.-H., Wadepohl, H. & Gade, L. H. Highly enantioselective copper-catalyzed electrophilic trifluoromethylation of β-ketoesters. J. Am. Chem. Soc. 134, 10769–10772 (2012).
Liu, J. et al. Enantioselective di-/perfluoroalkylation of β-ketoesters enabled by cooperative photoredox/nickel catalysis. Org. Lett. 20, 461–464 (2018).
Gao, X., Xiao, Y.-L., Wan, X. & Zhang, X. Copper-catalyzed highly stereoselective trifluoromethylation and difluoroalkylation of secondary propargyl sulfonates. Angew. Chem. Int. Ed. 57, 3187–3191 (2018).
Gu, Y., Lu, C., Gu, Y. & Shen, Q. Ligand-controlled copper-catalyzed highly regioselective difluoromethylation of allylic chlorides/bromides and propargyl bromides. Chin. J. Chem. 36, 55–58 (2018).
Gao, X., Cheng, R., Xiao, Y.-L., Wan, X.-L. & Zhang, X. Copper-catalyzed highly enantioselective difluoroalkylation of secondary propargyl sulfonates with difluoroenoxysilanes. Chem 5, 2987–2999 (2019).
Trost, B. M., Gholami, H. & Zell, D. Palladium-catalyzed asymmetric allylic fluoroalkylation/trifluoromethylation. J. Am. Chem. Soc. 141, 11446–11451 (2019).
An, L., Tong, F.-F., Zhang, S. & Zhang, X. Stereoselective functionalization of racemic cyclopropylzinc reagents via enantiodivergent relay coupling. J. Am. Chem. Soc. 142, 11884–11892 (2020).
Min, Q.-Q., Yin, Z., Feng, Z., Guo, W.-H. & Zhang, X. Highly selective gem-difluoroallylation of organoborons with bromodifluoromethylated alkenes catalyzed by palladium. J. Am. Chem. Soc. 136, 1230–1233 (2014).
You, S. L., Zhu, X. Z., Luo, Y. M., Hou, X. L. & Dai, L. X. Highly regio- and enantioselective palladium-catalyzed allylic alkylation and amination of monosubstituted allylic acetates with novel ferrocene P, N-ligands. J. Am. Chem. Soc. 123, 7471–7472 (2001).
Zheng, W. H., Sun, N. & Hou, X. L. Highly regio- and enantioselective palladium-catalyzed allylic alkylation and amination of dienyl esters with 1,1’-P,N-Ferrocene ligands. Org. Lett. 7, 5151–5154 (2005).
Zhang, K., Peng, Q., Hou, X.-L. & Wu, Y.-D. Highly enantioselective palladium-catalyzed alkylation of acyclic amides. Angew. Chem. Int. Ed. 47, 1741–1744 (2008).
Chen, J. P., Ding, C. H., Liu, W., Hou, X. L. & Dai, L. X. Palladium catalyzed regio-, diastereo-, and enantioselective allylic alkylation of acylsilanes with monosubstituted allyl substrates. J. Am. Chem. Soc. 132, 15493–15495 (2010).
Tarrant, P. & Lovelace, M. Free radical additions involving fluorine compounds. I. The addition of dibromodifluoromethane to hydrocarbon olefins. J. Am. Chem. Soc. 76, 3466–3468 (1954).
Seyferth, D., Simon, R. M., Sepelak, D. J. & Klein, H. A. gem-Difluoroallyllithium: improved synthesis brings improved applicability. J. Org. Chem. 45, 2273–2474 (1980).
Kirihara, M. et al. α-Bromo-α,α-difluoroallyl derivatives as synthetic intermediate: nucleophilic substitution of α-bromo-α,α-difluoroallyl derivatives in the presence of palladium catalysts. Chem. Pharm. Bull. 48, 885 (2000).
Fernandez, M., Uria, U., Vicario, J. L., Reyes, E. & Carrillo, L. Enantioselective conjugate addition of donor-acceptor hydrazones to α,β-unsaturated aldehydes through formal diaza-ene reaction: access to 1,4-dicarbonyl compounds. J. Am. Chem. Soc. 134, 11872–11875 (2012).
Kang, Q.-K. et al. Enantioselective alkylation of N-arylhydrazones derived from α-keto esters and isatin derivatives through asymmetric phase-transfer catalysis. Chem. Asian J. 13, 1780–1783 (2018).
Wang, Y., Wang, Q. & Zhu, J.-P. Organocatalytic nucleophilic addition of hydrazones to imines: synthesis of enantioenriched vicinal diamines. Angew. Chem. Int. Ed. 56, 5612–5615 (2017).
Zhu, D.-H. et al. Umpolung of carbonyl groups as alkyl organometallic reagent surrogates for palladium-catalyzed allylic alkylation. Angew. Chem. Int. Ed. 57, 16520–16524 (2018).
Lv, L. & Li, C.-J. Palladium-catalyzed defluorinative alkylation of gem-difluorocyclopropanes: switching regioselectivity via simple hydrazones. Angew. Chem. Int. Ed. 60, 13098–13104 (2021).
Keith, J. A. et al. The Inner-sphere process in the enantioselective Tsuji allylation reaction with (S)-t-Bu-phosphinooxazoline ligands. J. Am. Chem. Soc. 129, 11876–11877 (2007).
Zhang, P., Brozek, L. A. & Morken, J. P. Pd-catalyzed enantioselective allyl−allyl cross-coupling. J. Am. Chem. Soc. 132, 10686–10688 (2007).
Zhang, P., Le, H., Kyne, R. E. & Morken, J. P. Enantioselective construction of all-carbon quaternary centers by branch-selective Pd-catalyzed allyl–allyl cross-coupling. J. Am. Chem. Soc. 133, 9716–9719 (2011).
Brozek, L. A., Ardolino, M. J. & Morken, J. P. Diastereocontrol in asymmetric allyl–allyl cross-coupling: stereocontrolled reaction of prochiral allylboronates with prochiral allyl chlorides. J. Am. Chem. Soc. 133, 16778–16781 (2011).
Ardolino, M. J. & Morken, J. P. Congested C–C bonds by Pd-catalyzed enantioselective allyl–allyl cross-coupling, a mechanism-guided solution. J. Am. Chem. Soc. 136, 7092–7100 (2014).
Chen, J.-P., Peng, Q., Lei, B.-L., Hou, X.-L. & Wu, Y.-D. Chemo- and regioselectivity-tunable Pd-catalyzed allylic alkylation of imines. J. Am. Chem. Soc. 133, 14180–14183 (2011).
Bai, D.-C. et al. Palladium/N-heterocyclic carbene catalysed regio and diastereoselective reaction of ketones with allyl reagents via inner-sphere mechanism. Nat. Commun. 7, 11806 (2016).
Yu, F.-L. et al. Pd-catalyzed allylic alkylation of gem-alkyl, aryl-disubstituted allyl reagents with ketones: diastereoselective construction of vicinal tertiary and quaternary carbon centers. ACS Catal. 8, 3317–3321 (2018).
Zanda, M. Trifluoromethyl group: an effective xenobiotic function for peptide backbone modification. N. J. Chem. 28, 1401–1411 (2004).
Sani, M., Volonterio, A. & Zanda, M. The trifluoroethylamine function as peptide bond replacement. ChemMedChem 2, 1693–1700 (2007).
Onyeagusi, C. I. & Malcolmson, S. J. Strategies for the catalytic enantioselective synthesis of α trifluoromethyl amines. ACS Catal. 10, 12507–12536 (2020).
Cheng, R. et al. Highly γ-selective arylation and carbonylative arylation of 3-Bromo-3,3-difluoropropene via nickel catalysis. Angew. Chem. Int. Ed. 60, 12386–12391 (2021).
Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (22071143, 21532010), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20030100), the Chinese Academy of Sciences, the Technology Commission of Shanghai Municipality, and the Croucher Foundation of Hong Kong.
Author information
Authors and Affiliations
Contributions
S.H., C.-H.D., and X.-L.H. conceived and designed the experiments. S.H. performed the experiments. F.-F.T. performed some experiments and did the mechanism study. D.-C.B., G.-P.Z., Y.-J.J., and C.-H.D. revised the manuscript. B.Z. and Y.-L.G. conducted MS analysis. X.L. conducted XRD analysis. X.-L.W. conducted chiral HPLC analysis. X.Z. and X.-L.H. wrote the paper. All authors analyzed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, S., Tong, FF., Bai, DC. et al. Regio- and enantioselective umpolung gem-difluoroallylation of hydrazones via palladium catalysis enabled by N-heterocyclic carbene ligand. Nat Commun 12, 6551 (2021). https://doi.org/10.1038/s41467-021-26667-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-26667-0